This section deals with how atoms behave in
static electric fields. The method is straightforward,
involving second order perturbation
theory. The treatment describes the effects of symmetry on the
basic interaction, polarizability, and the concept of oscillator
strength.
Perturbation Theory of Polarizability
We will find the energy and polarizability of an atom in
a static field along the +z direction. We apply perturbation
theory taking  to describe the unperturbed atomic system and
to describe the unperturbed atomic system and

Non-degenerate eigenstates have to be eigenstates of parity. Since  is odd under parity operation, parity requires that
is odd under parity operation, parity requires that
 . So
the first order perturbation vanishes. To second order, the energy
is given by
. So
the first order perturbation vanishes. To second order, the energy
is given by

If we define now the polarizability in state  as
as
- {EQ_polarsix}

we obtain 
The dipole moment is the
expectation value of the dipole operator,
using the first order perturbed state vector.

![{\displaystyle =2{\rm {Re}}[\langle n^{(0)}|d|n^{(1)}\rangle ]=2e^{2}{\rm {Re}}{\left[\sum _{s,m}{\frac {\langle n^{(0)}|s|m\rangle \langle m|z|n^{(0)}\rangle }{E_{m}-E_{n}}}\right]}{\hat {s}}\cdot {\hat {z}}{\mathcal {E}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/afd33952a21990436ab03840a4b4e1e286b70684)
where the sum is over  Only the term
Only the term  will
contribute, and we can express the induced dipole moment by the polarizability:
will
contribute, and we can express the induced dipole moment by the polarizability:

Note that the Stark shift is
 and not equal to
and not equal to
 .
.  is the expectation value for the electrostatic potential energy of the dipole moment, but the total energy change is only one half of this since energy is needed to admix excited states into the ground state.
is the expectation value for the electrostatic potential energy of the dipole moment, but the total energy change is only one half of this since energy is needed to admix excited states into the ground state.
Note that polarizability has the dimensions of length , i.e. volume.
As an example, for the ground state of hydrogen we can obtain a
lower limit for the polarizability by considering only the
contribution to the sum of the
, i.e. volume.
As an example, for the ground state of hydrogen we can obtain a
lower limit for the polarizability by considering only the
contribution to the sum of the  state. Values for the various
moments in hydrogen are given in Bethe and Salpeter, Section 63.
Using
state. Values for the various
moments in hydrogen are given in Bethe and Salpeter, Section 63.
Using  = 1.666, and
= 1.666, and  , we obtain
, we obtain  atomic units (i.e.
atomic units (i.e.  ).
).
The polarizability of the ground state of hydrogen can be
calculated exactly. It turns out that the  state makes the
major contribution, and that the higher bound states contribute
relatively little. However, the continuum makes a significant
contributions. The exact value is 4.5.
state makes the
major contribution, and that the higher bound states contribute
relatively little. However, the continuum makes a significant
contributions. The exact value is 4.5.
To put the above result for the polarizability in perspective, note that the potential
of a conducting sphere of radius  in a uniform electric field
in a uniform electric field
 is given by
is given by

The induced dipole moment is  , so that the
polarizability is
, so that the
polarizability is  . For the ground state of hydrogen,
. For the ground state of hydrogen,
 , so to a crude approximation, in an electric
field hydrogen behaves like a conducting sphere.
, so to a crude approximation, in an electric
field hydrogen behaves like a conducting sphere.
Polarizability may be approximated easily, though not accurately,
using Unsold's approximation in which the energy term in the
denominator of Eq. \ref{EQ_polarsix} is replaced by an average
energy interval  . The sum can then be
evaluated using the closure rule
. The sum can then be
evaluated using the closure rule  . (Note
that the term
. (Note
that the term  does not need to be excluded from the sum,
since
does not need to be excluded from the sum,
since  .). With this approximation,
.). With this approximation,

For hydrogen in the ground state,  . If we
take the average excitation energy to be
. If we
take the average excitation energy to be  ,
the result is
,
the result is  .
.
Beyond the quadratic Stark effect
It should be obvious from the previous discussion that the Stark effect for a state of  is quadratic only when
is quadratic only when

|
(EQ_ beyondone)
|
when  is the nearest state of opposite parity to
is the nearest state of opposite parity to  .
.
If  is the ground state, we can expect
is the ground state, we can expect 
 Hartree and
Hartree and  (virial theorem). Hence the Stark shift should be quadratic if the field is well below the critical value
(virial theorem). Hence the Stark shift should be quadratic if the field is well below the critical value

|
(EQ_ beyondtwo)
|
[ is atomic unit of field]
is atomic unit of field]  —a field three orders of magnitude in excess of what can be produced in a laboratory except in a vanishingly small volume.
—a field three orders of magnitude in excess of what can be produced in a laboratory except in a vanishingly small volume.
If  is an excited state, say
is an excited state, say  , this situation changes <it>
dramatically
</it>. In general, the matrix element
, this situation changes <it>
dramatically
</it>. In general, the matrix element  and
and  to the next level of opposite parity depends on the quantum defect:
to the next level of opposite parity depends on the quantum defect:

|
(EQ_ beyondthree)
|
Thus the critical field is lowered to


|
(EQ_ beyondfour)
|
Considering that quantum defects are typically  when
when  is the largest
is the largest  of an electron in the core), it is clear that even 1 V/cm fields will exceed
of an electron in the core), it is clear that even 1 V/cm fields will exceed  for higher
for higher  levels if
levels if  . Large laboratory fields (
. Large laboratory fields ( V/cm) can exceed
V/cm) can exceed  even for
even for  states if
states if  .
.
When the electric field exceeds  states with different
states with different  but the same
but the same  are degenerate to the extent that their quantum defects are small. Once
are degenerate to the extent that their quantum defects are small. Once  exceeds the number of core electrons, these states will easily become completely mixed by the field and they must be diagonalized exactly. The result is eigenstates possessing apparently permanent electric dipoles with a resulting linear Stark shift (see following figure). As the field increases, these states spread out in energy. First they run into states with the same
exceeds the number of core electrons, these states will easily become completely mixed by the field and they must be diagonalized exactly. The result is eigenstates possessing apparently permanent electric dipoles with a resulting linear Stark shift (see following figure). As the field increases, these states spread out in energy. First they run into states with the same  but different quantum defects; then the groups of states with different
but different quantum defects; then the groups of states with different  begin to overlap. At this point a matrix containing all
begin to overlap. At this point a matrix containing all  states with
states with  greater or equal to
greater or equal to  must be diagonalized. The only saving grace is that the lowest
must be diagonalized. The only saving grace is that the lowest  states do not partake in this strong mixing; however, the
states do not partake in this strong mixing; however, the  states near the continuum always do if there is an
states near the continuum always do if there is an  -field present.\
-field present.\
The situation described above differs qualitatively for hydrogen since it has no quantum defects and the energies are degenerate. In this case the zero-field problem may be solved using a basis which diagonalizes the Hamiltonian both for the atom above and also in the presence of an electric field. This approach corresponds to solving the H atom in parabolic–ellipsoidal coordinates and results in the presence of an integral quantum number which replaces  . The resulting states possess permanent dipole moments which vary with this quantum number and therefore have linear Stark effects even in infinitesimal fields. Moreover the matrix elements which mix states from different
. The resulting states possess permanent dipole moments which vary with this quantum number and therefore have linear Stark effects even in infinitesimal fields. Moreover the matrix elements which mix states from different  manifolds vanish at all fields, so the upper energy levels from one manifold cross the lower energy levels from the manifold above without interacting with them.\
manifolds vanish at all fields, so the upper energy levels from one manifold cross the lower energy levels from the manifold above without interacting with them.\
The following example shows the high field stark effect for Li. Only the  term in Li has an appreciable quantum defect, and it has been suppressed by selecting final states with
term in Li has an appreciable quantum defect, and it has been suppressed by selecting final states with  .
.
The dramatic difference between the physical properties of atoms with  and the properties of the same atoms in their ground state, coupled with the fact that these properties are largely independent of the type of atom which is excited, justifies the application of the name Rydberg atoms to highly excited atoms in general.\
and the properties of the same atoms in their ground state, coupled with the fact that these properties are largely independent of the type of atom which is excited, justifies the application of the name Rydberg atoms to highly excited atoms in general.\
File:06-E-FIELD/Stark pattern.eps Stark effect and field ionization in Li for levels with

. Each vertical line represents a measurement at that field of the number of atoms excited (from the

state) by radiation whose energy falls the indicated amount below the ionization limit. Thus the patterns made by absorption peaks at successive field strengths represent the behavior of the energy levels with increasing field. At zero field the levels group according to the principal quantum number

; at intermediate field the levels display a roughly linear Stark effect, and at high fields they disappear owing to field ionization. The solid line is the classically predicted ionization field (see next section). Figure taken from Littman, Kash and Kleppner.
Field ionization
If an atom is placed in a sufficiently high electric field it will be ionized, a process called <it>
field ionization
</it>. An excellent order of magnitude estimate of the field  , required to ionize an atom which is initially in a level bound by energy
, required to ionize an atom which is initially in a level bound by energy  can be obtained by the following purely classical argument: the presence of the field adds the term
can be obtained by the following purely classical argument: the presence of the field adds the term  to the potential energy of the atom. This produces a potential with a maximum
to the potential energy of the atom. This produces a potential with a maximum  and the atom will ionize if
and the atom will ionize if  .
.
The figure shows the combined potential as well as  and
and 

|
(EQ_ fieldionone)
|
The appropriate maximum occurs at

|
(EQ_ fieldiontwo)
|
as determined from  . Equating
. Equating  and
and  gives
gives

|
(EQ_ fieldionthree)
|
for level with energy  and quantum number
and quantum number  .
.
The predictions of this formula for  is usually accurate within 20% in spite of its neglect of both quantum tunneling and the change in
is usually accurate within 20% in spite of its neglect of both quantum tunneling and the change in  produced by the field. [This latter deficiency is remedied in the comparison with Li data shown in the preceding part of this section because the eye naturally uses the ionization field appropriate to the perturbed energy of the state rather than its zero-field energy.] Tunneling manifests itself as a finite decay rate for states which classically lie lower than the barrier. The increase of the ionization rate with field is so dramatic, however, that the details of the experiment do not influence the field at which ionization occurs very much: calculations [u'BHR65'] show the ionization rate increasing from
produced by the field. [This latter deficiency is remedied in the comparison with Li data shown in the preceding part of this section because the eye naturally uses the ionization field appropriate to the perturbed energy of the state rather than its zero-field energy.] Tunneling manifests itself as a finite decay rate for states which classically lie lower than the barrier. The increase of the ionization rate with field is so dramatic, however, that the details of the experiment do not influence the field at which ionization occurs very much: calculations [u'BHR65'] show the ionization rate increasing from  /sec to
/sec to  /sec for a 30% increase in the field.\
/sec for a 30% increase in the field.\
Oddly enough the classical prediction works worse for H than for any other atom. This is a reflection of the fact that certain matrix elements necessary to mix the  states (so the wave function samples the region near
states (so the wave function samples the region near  ) are rigorously zero in H, as discussed in the preceding part of this section. Hence the orbital ellipse of the electron does not precess and can remain on the side of the nucleus. There its energy will increase with
) are rigorously zero in H, as discussed in the preceding part of this section. Hence the orbital ellipse of the electron does not precess and can remain on the side of the nucleus. There its energy will increase with  , but it will not spill over the lip of the potential and ionize.
, but it will not spill over the lip of the potential and ionize.
Atoms in an Oscillating Electric Field
There is a close connection between the behavior of an atom in a
static electric field and its response to an oscillating field,
i.e. a connection between the Stark effect and radiation
processes. In the former case, the field induces a static dipole
moment; in the latter case, it induces an oscillating moment. An
oscillating moment creates an oscillating macroscopic
polarization and leads to the absorption and emission of
radiation. We shall calculate the response of an atom to an
oscillating field

where  is the polarization vector for the field. For
a weak field the time varying state
of this system can be found from first order time dependent
perturbation theory. We shall write
the electric dipole operator as D = -er. (This is a
change of notation. Previously the
symbol was d.) The Hamiltonian naturally separates into two
parts,
is the polarization vector for the field. For
a weak field the time varying state
of this system can be found from first order time dependent
perturbation theory. We shall write
the electric dipole operator as D = -er. (This is a
change of notation. Previously the
symbol was d.) The Hamiltonian naturally separates into two
parts,  ,
where
,
where  is the unperturbed Hamiltonian and
is the unperturbed Hamiltonian and

We shall express the solution of the time dependent Schroedinger
equation in terms of the
eigenstates of  .
.

where  . Because of the perturbation
. Because of the perturbation
 , the
, the  's become time
dependent, and we have
's become time
dependent, and we have

Left multiplying the final two expressions by  to
project out the
to
project out the  -th terms yields
-th terms yields

where  . In perturbation theory,
this set of equations is solved
by a set of approximations to
. In perturbation theory,
this set of equations is solved
by a set of approximations to  labeled
labeled  .
Starting with
.
Starting with

one sets

and solves for the successive approximations by integration.
We now apply this to the problem of an atom which is in its ground
state  at
at  , and which is
subject to the interaction of Eq.\ \ref{EQ_atomoef2}. Consequently
, and which is
subject to the interaction of Eq.\ \ref{EQ_atomoef2}. Consequently
 ,
,  . Substituting in Eq.\ \ref{EQ_atomoef7} and integrating
from
. Substituting in Eq.\ \ref{EQ_atomoef7} and integrating
from  to
to  gives
gives
![{\displaystyle {\begin{array}{rcl}a_{k}^{(1)}(t)&=&(i\hbar )^{-1}\int _{0}^{t}dt^{\prime }\langle k|H^{\prime }(t^{\prime })|g\rangle e^{i\omega _{kg}t^{\prime }}\\&=&-(i\hbar )^{-1}\langle k|{\hat {e}}\cdot D|g\rangle {\frac {\mathcal {E}}{2}}\int _{0}^{t}dt^{\prime }{\left[e^{i(\omega _{kg}+\omega )t^{\prime }}+e^{i(\omega _{kg}-\omega )t^{\prime }}\right]}\\&=&{\frac {\mathcal {E}}{2\hbar }}\langle k|{\hat {e}}\cdot D|g\rangle {\left[{\frac {e^{i(\omega _{kg}+\omega )t}-1}{\omega _{kg}+\omega }}+{\frac {e^{i(\omega _{kg}-\omega )t}-1}{\omega _{kg}-\omega }}\right]}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/37d5e4872c67deeb9e95cc30b7f74ecd1122ce0f)
The -1 terms in the square bracketed term arises because it is
assumed that the field was turned on
instantaneously at  . They represent transients that rapidly
damp and can be neglected.
. They represent transients that rapidly
damp and can be neglected.
The term with  , in the denominator is the
counter-rotating term. It can be
neglected if one is considering cases where
, in the denominator is the
counter-rotating term. It can be
neglected if one is considering cases where  (i.e. near resonance), but
we shall retain both terms and calculate the expectation value of
the first order time dependent
dipole operator
(i.e. near resonance), but
we shall retain both terms and calculate the expectation value of
the first order time dependent
dipole operator 
![{\displaystyle {\begin{array}{rcl}\langle D(\omega ,t)\rangle &=&2{\rm {Re}}{\left\{\langle g|{\bf {D}}|\sum _{k}a_{k}^{(1)}(t)e^{-i\omega _{kg}}|k\rangle \right\}}\\&=&{\mathcal {E}}{\rm {Re}}{\left[\sum _{k}{\frac {\langle g|D|k\rangle \langle k|{\hat {e}}\cdot D|g\rangle }{\hbar }}{\left\{{\frac {e^{i\omega t}}{\omega _{kg}+\omega }}+{\frac {e^{-i\omega t}}{\omega _{kg}-\omega }}\right\}}\right]}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/50bd4119db9415e85baec8331b26488d24624bfc)
If we consider the case of linearly polarized light  , then
, then

We can write  in terms of a polarizability
in terms of a polarizability  :
:

This result diverges if  . Later,
when we introduce radiative damping, the divergence will be
avoided in the usual way.
. Later,
when we introduce radiative damping, the divergence will be
avoided in the usual way.
Oscillator Strength
Eq.\ \ref{EQ_atomoef11} resemble the oscillating dipole moment of a
system of classical
oscillators. Consider a set of oscillators having charge  ,
mass
,
mass  , and natural frequency
, and natural frequency
 , driven by the field
, driven by the field  . The
amplitude of the motion is given
by
. The
amplitude of the motion is given
by

If we have a set of such oscillators, then the total oscillating
moment is given by

This is strongly reminiscent of Eq.\ \ref{EQ_atomoef10}. It is
useful to introduce the concept of
oscillator strength, a dimensionless quantity defined as

where  and
and  are any two eigenstates. Note that
are any two eigenstates. Note that
 is positive if
is positive if  , i.e. for absoprtion, and negative if
, i.e. for absoprtion, and negative if
 Then, Eq.\ \ref{EQ_atomoef10} becomes
Then, Eq.\ \ref{EQ_atomoef10} becomes

Comparing this with Eq.\ \ref{EQ_ostre2}, we see that the behavior
of an atom in an oscillating
field mimics a set of classical oscillators with the same
frequencies as the eigenfrequencies of
the atom, but having effective charge strengths  .\\
.\\
The oscillator strength is useful for characterizing radiative
interactions and also the
susceptibiltiy of atoms. It satisfies an important sum rule, the
Thomas-Reiche-Kuhn sum rule:

We prove by considering the general Hamiltonian

Using the commutator relation
![{\displaystyle [A,B^{2}]=[A,B]B+B[A,B],}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5e16bcf41c2c00f41091a8b2f6ec1a54c3c26aa3)
and the relation ![{\displaystyle [r_{j},p_{k}]=i\hbar \delta _{jk}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b47639ff8ac69b050c5dc9d8cf58f9c6d17af6a3) , we have
, we have
![{\displaystyle [r,H]={\frac {i\hbar }{m}}p}](https://wikimedia.org/api/rest_v1/media/math/render/svg/335156c1e95f556b5ff6979d61bd8c1ef0ea7263)
where  , and
, and  .
However,
.
However,
![{\displaystyle \langle j|[r,H]|k\rangle =(E_{k}-E_{n})\langle j|r|k\rangle }](https://wikimedia.org/api/rest_v1/media/math/render/svg/4756f9f0266fc68ae7c3ce2a7995ae930e09add7)
Consequently,

where  . Thus, we can write
Eq.\ \ref{EQ_ostre3} in either of two forms:
. Thus, we can write
Eq.\ \ref{EQ_ostre3} in either of two forms:

Taking half the sum of these equations and using the closure
relation  , we have
, we have
![{\displaystyle \sum _{k}f_{kj}={\frac {i}{\hbar }}{\left[\langle j|p_{z}z-zp_{z}|j\rangle \right]}=1}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9256c4cbcfdc8f1d87d37c52474b7279a2ec1990)
We have calculated this for a one-electron atom, but the
application to a Z-electron atom is
straightforward because the Hamiltonian in Eq.\ \ref{EQ_ostre6}
is quite general. In this case

Here  is some eigenstate of the system, and the index
is some eigenstate of the system, and the index  describes all the eigenstates of all
the electrons -- including continuum states. In cases where only
a single electron will be
excited, however, for instance in the optical regime of a
"single-electron" atom where the inner core
electrons are essentially unaffected by the radiation, the atom
behaves as if it were a single electron system with
describes all the eigenstates of all
the electrons -- including continuum states. In cases where only
a single electron will be
excited, however, for instance in the optical regime of a
"single-electron" atom where the inner core
electrons are essentially unaffected by the radiation, the atom
behaves as if it were a single electron system with  .
.
Note that  is positive if
is positive if  , i.e. if the final
state lies above the initial state. Such a transition corresponds to
absorption of a photon. Since
, i.e. if the final
state lies above the initial state. Such a transition corresponds to
absorption of a photon. Since  , the oscillator strength
for emission of a photon is negative.
, the oscillator strength
for emission of a photon is negative.
Our definition of oscillator strength, Eq.\ \ref{EQ_ostre3},
singles out a particular axis, the  -axis, fixed by the
polarization of the light. Consequently, it depends on the orientation
of the atom in the initial state and final states. It is convenient to
introduce the average oscillator
strength (often simply called the oscillator strength), by
letting
-axis, fixed by the
polarization of the light. Consequently, it depends on the orientation
of the atom in the initial state and final states. It is convenient to
introduce the average oscillator
strength (often simply called the oscillator strength), by
letting  , summing over the initial
, summing over the initial  state and averaging over the
final state.\\
state and averaging over the
final state.\\

(This is the conversion followed by Sobelman.) It is evident that

where  is the multiplicity factor for state
is the multiplicity factor for state  . An
extensive discussion of the sum rules and
their applications to oscillator strengths and transition
momentums can be found in Bethe and
Salpeter, section 6.1. Among the interesting features they point
out is that transitions from an
initial state
. An
extensive discussion of the sum rules and
their applications to oscillator strengths and transition
momentums can be found in Bethe and
Salpeter, section 6.1. Among the interesting features they point
out is that transitions from an
initial state  to a final state
to a final state  on the average have
stronger oscillator strengths for absorption if
on the average have
stronger oscillator strengths for absorption if  , and stronger oscillator
strengths for emission if
, and stronger oscillator
strengths for emission if  . In other words,
atoms "like" to increase their
angular momentum on absorption of a photon, and decrease it on
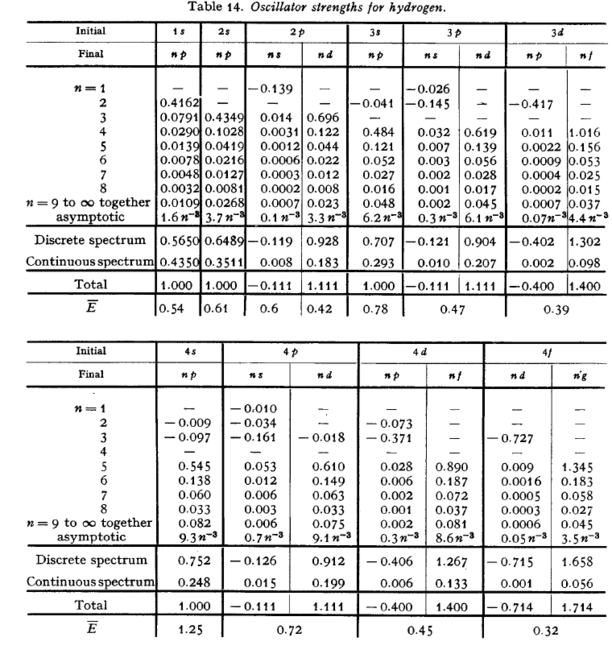
emission. The following page gives
a table of oscillator strengths for hydrogen in which this
tendency can be readily identified.
(Taken from {\it The Quantum Mechanics of One- and Two-Electron Atoms}, H.A. Bethe and E.E. Salpeter, Academic Press (1957).)
. In other words,
atoms "like" to increase their
angular momentum on absorption of a photon, and decrease it on
emission. The following page gives
a table of oscillator strengths for hydrogen in which this
tendency can be readily identified.
(Taken from {\it The Quantum Mechanics of One- and Two-Electron Atoms}, H.A. Bethe and E.E. Salpeter, Academic Press (1957).)
\caption{
Oscillator strengths for hydrogen. From
Mechanics of One- and Two-Electron Atoms}
Index of refraction
As an application of the expression for the ac polarizability, we now discuss the index of refraction of an atomic gas. What we derive here, is fully sufficient to understand both absorption imaging and phase-contrast imaging used to observe ultracold atomic clouds.

In the case of near resonant light we can neglect the counter-rotating term, and let 

Define the natural linewidth (this expression will be derived later in the course)

So we can rewrite the expression for the refractive index:


where, since

we have

In our derivation of the polarizability, we didn't include any damping. The effect of damping is to give the refractive index an imaginary (absorptive) part. Damping can be included by adding an imaginary part to the detuning  :
:


![{\displaystyle -{\frac {\Gamma }{2\delta }}\rightarrow {\frac {\Gamma }{\gamma }}{\bigg (}{\frac {-1}{\delta '+i}}{\bigg )}={\frac {\Gamma }{\gamma }}{\bigg [}{\frac {i}{1+\delta '^{2}}}-{\frac {\delta '}{1+\delta '^{2}}}{\bigg ]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/eb78543c9cc15115ec953d0e8e9cd07c77f655be)
where the first term in brackets corresponds to dispersion and the second to absorption. This become obvious when one propagates the wave with the wavevector modified by the index of refraction:
![{\displaystyle e^{ikz}=e^{in_{r}{\frac {\omega }{c}}z}=e^{-{\frac {D_{0}}{2}}[{\frac {1}{1+\delta '^{2}}}-{\frac {i\delta '}{1+\delta '^{2}}}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/72317571d524b12432dc20f8849a006278731164)
The optical density on resonance is:

Note: When the linewidth is determined by spontaneous emission then 

the maximum phase shift is at 

References









![{\displaystyle =2{\rm {Re}}[\langle n^{(0)}|d|n^{(1)}\rangle ]=2e^{2}{\rm {Re}}{\left[\sum _{s,m}{\frac {\langle n^{(0)}|s|m\rangle \langle m|z|n^{(0)}\rangle }{E_{m}-E_{n}}}\right]}{\hat {s}}\cdot {\hat {z}}{\mathcal {E}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/afd33952a21990436ab03840a4b4e1e286b70684)
































































































![{\displaystyle {\begin{array}{rcl}a_{k}^{(1)}(t)&=&(i\hbar )^{-1}\int _{0}^{t}dt^{\prime }\langle k|H^{\prime }(t^{\prime })|g\rangle e^{i\omega _{kg}t^{\prime }}\\&=&-(i\hbar )^{-1}\langle k|{\hat {e}}\cdot D|g\rangle {\frac {\mathcal {E}}{2}}\int _{0}^{t}dt^{\prime }{\left[e^{i(\omega _{kg}+\omega )t^{\prime }}+e^{i(\omega _{kg}-\omega )t^{\prime }}\right]}\\&=&{\frac {\mathcal {E}}{2\hbar }}\langle k|{\hat {e}}\cdot D|g\rangle {\left[{\frac {e^{i(\omega _{kg}+\omega )t}-1}{\omega _{kg}+\omega }}+{\frac {e^{i(\omega _{kg}-\omega )t}-1}{\omega _{kg}-\omega }}\right]}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/37d5e4872c67deeb9e95cc30b7f74ecd1122ce0f)



![{\displaystyle {\begin{array}{rcl}\langle D(\omega ,t)\rangle &=&2{\rm {Re}}{\left\{\langle g|{\bf {D}}|\sum _{k}a_{k}^{(1)}(t)e^{-i\omega _{kg}}|k\rangle \right\}}\\&=&{\mathcal {E}}{\rm {Re}}{\left[\sum _{k}{\frac {\langle g|D|k\rangle \langle k|{\hat {e}}\cdot D|g\rangle }{\hbar }}{\left\{{\frac {e^{i\omega t}}{\omega _{kg}+\omega }}+{\frac {e^{-i\omega t}}{\omega _{kg}-\omega }}\right\}}\right]}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/50bd4119db9415e85baec8331b26488d24624bfc)





















![{\displaystyle [A,B^{2}]=[A,B]B+B[A,B],}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5e16bcf41c2c00f41091a8b2f6ec1a54c3c26aa3)
![{\displaystyle [r_{j},p_{k}]=i\hbar \delta _{jk}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b47639ff8ac69b050c5dc9d8cf58f9c6d17af6a3)
![{\displaystyle [r,H]={\frac {i\hbar }{m}}p}](https://wikimedia.org/api/rest_v1/media/math/render/svg/335156c1e95f556b5ff6979d61bd8c1ef0ea7263)


![{\displaystyle \langle j|[r,H]|k\rangle =(E_{k}-E_{n})\langle j|r|k\rangle }](https://wikimedia.org/api/rest_v1/media/math/render/svg/4756f9f0266fc68ae7c3ce2a7995ae930e09add7)




![{\displaystyle \sum _{k}f_{kj}={\frac {i}{\hbar }}{\left[\langle j|p_{z}z-zp_{z}|j\rangle \right]}=1}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9256c4cbcfdc8f1d87d37c52474b7279a2ec1990)
























![{\displaystyle -{\frac {\Gamma }{2\delta }}\rightarrow {\frac {\Gamma }{\gamma }}{\bigg (}{\frac {-1}{\delta '+i}}{\bigg )}={\frac {\Gamma }{\gamma }}{\bigg [}{\frac {i}{1+\delta '^{2}}}-{\frac {\delta '}{1+\delta '^{2}}}{\bigg ]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/eb78543c9cc15115ec953d0e8e9cd07c77f655be)
![{\displaystyle e^{ikz}=e^{in_{r}{\frac {\omega }{c}}z}=e^{-{\frac {D_{0}}{2}}[{\frac {1}{1+\delta '^{2}}}-{\frac {i\delta '}{1+\delta '^{2}}}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/72317571d524b12432dc20f8849a006278731164)




