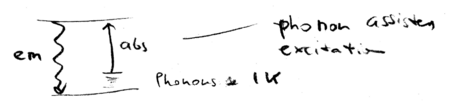imported>Ichuang |
imported>Ichuang |
| Line 9: |
Line 9: |
| | == Optical Molasses == | | == Optical Molasses == |
| | | | |
| − | === The spontaneous light force ===
| + | {{:Optical Molasses} |
| − | Consider a two-level atom with energy spacing <math>\omega_0</math>,
| |
| − | interacting with a single mode laser beam:
| |
| − | ::[[Image:chapter1-intro-to-cooling-Lec1-twolevel.png|thumb|408px|none|]]
| |
| − | Let the laser intensity be <math>I</math>, and the interaction matrix element
| |
| − | between atom and light be <math>\hbar\omega_R = \vec{E}\cdot
| |
| − | | \langle 1|e\vec{r}|2{\rangle}|</math>, where <math>\omega_R</math> is known as the Rabi frequency,
| |
| − | <math>\vec{E}</math> is the electric field strength, and <math>e\vec{r}</math> is the dipole
| |
| − | moment of the atom.
| |
| − | | |
| − | It is useful to define a quantity known as the saturation intensity
| |
| − | <math>I_0</math> as the intensity of light at which the rabi frequency becomes
| |
| − | <math>\omega_R = \Gamma/\sqrt{2}</math>, where <math>\Gamma</math> is the spontaneous
| |
| − | emission rate (the natural decay rate of the atom from <math>|2{\rangle}</math> to
| |
| − | <math>|1{\rangle}</math>, excited to ground state). This gives
| |
| − | :<math>
| |
| − | \frac{I}{I_0} = \frac{2\omega_R^2}{\Gamma^2}
| |
| − | \,.
| |
| − | </math>
| |
| − | The rate at which photons are scattered from the atom is known to be
| |
| − | :<math>
| |
| − | \gamma_s = \frac{\Gamma}{2} \frac{I/I_0}{1+\frac{I}{I_0} +
| |
| − | (2\delta/\Gamma)^2}
| |
| − | \,,
| |
| − |
| |
| − | </math>
| |
| − | where <math>\delta</math> is the frequency detuning of the laser from the center
| |
| − | of resonance of the atom. Two useful limits of this scattering rate
| |
| − | are
| |
| − | :<math>\begin{array}{rcl}
| |
| − | \lim_{I\rightarrow\infty} \gamma_s &=& \frac{\Gamma}{2}
| |
| − | = \frac{1}{2\tau}
| |
| − | \\
| |
| − | \lim_{I\rightarrow I_0} \gamma_s &=& \frac{\Gamma}{4}
| |
| − | \,,
| |
| − | \end{array}</math>
| |
| − | where we have assumed <math>\delta=0</math> (resonant light). These expressions
| |
| − | have a natural physical interpretation: in the limit of infinite
| |
| − | intensity, the atomic levels become equally populated between the
| |
| − | excited and ground state, and thus only half the atoms (the excited
| |
| − | ones) can scatter light. Thus, the scattering rate is <math>\Gamma/2</math> in
| |
| − | that limit.
| |
| − | | |
| − | Suppose the force imparted by light on the atom is given by the recoil
| |
| − | of photons spontaneously emitted from the atom. This force would then
| |
| − | be
| |
| − | :<math>
| |
| − | F = \hbar k \gamma_s
| |
| − | \,,
| |
| − | </math>
| |
| − | where <math>\hbar k</math> is the momentum of each photon. This expression makes
| |
| − | several assumptions: that is is the net momentum transfer in absorption,
| |
| − | that there is no "stimulated" force, and that <math>\hbar k/m k \ll
| |
| − | \Gamma</math>, meaning that the jump in the Doppler shift is less than the
| |
| − | natural linewidth.
| |
| − | | |
| − | Typically, for alkali atoms, this force is <math>F\leq 10^5 g</math> times the
| |
| − | mass of an atom (<math>g</math> being the acceleration due to gravity). This
| |
| − | means that light can stop a sodium atom going at <math>1000</math> m/s in one
| |
| − | millisecond, or about half a meter. In comparison to electrostatic
| |
| − | forces on ions, this is very small, however: it is comparable to the
| |
| − | force exerted by an electric field of 1 millivolt/cm on an ionized
| |
| − | sodium atom.
| |
| − | | |
| − | Moving atoms experience a Doppler shift, which we can model as a
| |
| − | frequency dependent force, based on Eq.(\ref{eq:ci:lorentzian}), as
| |
| − | :<math>
| |
| − | F = \hbar k \frac{\Gamma}{2} \frac{I/I_0}{1+\frac{I}{I_0} +
| |
| − | 2\left[\frac{(2\delta+kv)}{\Gamma}\right]^2}
| |
| − | \,,
| |
| − |
| |
| − | </math>
| |
| − | where <math>v</math> is the velocity of the atom. The effect of a fixed laser
| |
| − | frequency on an ensemble of atoms is to modify their Maxwell-Boltzmann
| |
| − | thermal velocity distribution:
| |
| − | ::[[Image:chapter1-intro-to-cooling-l1fig2.png|thumb|408px|none|]]
| |
| − | Note how the initial distribution changes to one with atoms piling up
| |
| − | below the velocity group resonant with the laser. The atoms bunch.
| |
| − | Historically, this is the first method that was done to cool atoms to
| |
| − | Kelvin temperatures.
| |
| − | | |
| − | === One-dimensional optical molasses ===
| |
| − | Let us now turn to a method which allows cooling of atoms to zero
| |
| − | velocity. Consider two laser beams incident on an atom from opposite
| |
| − | directions. We assume that the total force is the sum of the two
| |
| − | forces, ignore standing wave effects, and take the laser intensity to
| |
| − | be low compared with the saturation intensity, <math>I\ll I_0</math>. Taking the
| |
| − | force to be the sum of two forces described by Eq.(\ref{eq:ci:vdf}),
| |
| − | we find that the two lorentzians sum to give the following force as a
| |
| − | function of velocity:
| |
| − | | |
| − | ::[[Image:20090410-094308_light-force-fig2.png|thumb|500px|none|]]
| |
| − | The velocity dependent force is positive from one light beam, and
| |
| − | negative from the other. With a detuning chosen such that force is
| |
| − | zero at zero velocity, the force around <math>v=0</math> can be expanded
| |
| − | linearly, giving
| |
| − | :<math>
| |
| − | F(v) = -\alpha v
| |
| − | \,,
| |
| − | </math>
| |
| − | where <math>\alpha</math> describes the viscosity imparted by the light
| |
| − | force to the atom, reflecting the restoring force applied when the
| |
| − | atom is not at zero velocity. This configuration is known as an {\em
| |
| − | optical molasses}, because of this restoring force, which makes the
| |
| − | light behave like a thick, viscous medium for the atoms in it.
| |
| − | The damping coefficient <math>\alpha</math> can be calculated to be
| |
| − | :<math>
| |
| − | \alpha = 2\hbar k^2
| |
| − | \frac{(2I/I_0)(2\delta/\Gamma)}{\left(1+\left(\frac{2\delta}{\Gamma}\right)^2\right)^2}
| |
| − | \,.
| |
| − | </math>
| |
| − | | |
| − | === The Doppler cooling limit ===
| |
| − | | |
| − | We have seen that the spontaneous light force, characterized by the
| |
| − | Lorentzian response of an atom to light, together with the Doppler
| |
| − | shift due to movement of the atom, gives a velocity dependent force,
| |
| − | which can be zero at zero velocity. Does this mean that the atoms can
| |
| − | be cooled to zero temperature?
| |
| − | | |
| − | If the rate of energy loss due to cooling is
| |
| − | :<math>
| |
| − | \dot{E}_{\rm cool} = F v = -\alpha v^2 = -\frac{2\alpha}{M} E
| |
| − | \,,
| |
| − | </math>
| |
| − | then we should reach zero velocity, and zero temperature. Indeed, the
| |
| − | kinetic energy decays exponentially. However, the spontaneous force
| |
| − | has a random character, and thus has fluctuations which limit the
| |
| − | minimum temperature achievable.
| |
| − | | |
| − | ==== Momentum diffusion limit ====
| |
| − | | |
| − | This limit is determined by momentum diffusion. The force imparted
| |
| − | can be described by a random walk. The final momentum is
| |
| − | :<math>
| |
| − | p_{\rm final}^{RMS} = \hbar k \sqrt{N}
| |
| − | \,,
| |
| − | </math>
| |
| − | on average, due to the random walk. Note that the momentum spread is
| |
| − | :<math>\begin{array}{rcl}
| |
| − | \langle p^2 \rangle &=& (\hbar k)^2 v
| |
| − | \\
| |
| − | \frac{d \langle p^2{\rangle}}{dt} &=& (\hbar k)^2 \gamma_s
| |
| − | \,.
| |
| − | \end{array}</math>
| |
| − | This describes heating which arises due to photons randomly scattering
| |
| − | in all directions, such that the net momentum almost adds up to zero,
| |
| − | but not quite. There is also a similar term due to absorption: some
| |
| − | atoms will absorb more or less photons, due to the Poissonian
| |
| − | statistics of absorption.
| |
| − | | |
| − | ==== Time variation of kinetic energy ====
| |
| − | | |
| − | Thus, the time variation of the kinetic energy due to the fluctuating
| |
| − | forces is
| |
| − | :<math>
| |
| − | \dot{E}_{\rm heat} = \frac{2\hbar^2 k^2\gamma_s}{2M} = \frac{D}{M}
| |
| − | \,,
| |
| − | </math>
| |
| − | where <math>D</math> is the momentum diffusion coefficient
| |
| − | :<math>
| |
| − | D = \frac{ \langle p^2{\rangle}}{2}
| |
| − | \,,
| |
| − | </math>
| |
| − | which we'll later see is a correlation function of the fluctuation
| |
| − | forces.
| |
| − | | |
| − | ==== Balance of heating and cooling ====
| |
| − | | |
| − | Let us now derive the Doppler limit for cooling. In equilibrium,
| |
| − | <math>\dot{E}_{heat} = \dot{E}_{cool}</math>. This means
| |
| − | :<math>
| |
| − | \frac{D}{M} = \frac{2\alpha}{M}E
| |
| − | \,.
| |
| − | </math>
| |
| − | The heating rate is independent of kinetic energy, whereas the cooling
| |
| − | rate is a function of kinetic energy. So as the atoms cool down, the
| |
| − | cooling rate slows down, resulting in a final temperature equilibrium
| |
| − | being reached:
| |
| − | :<math>
| |
| − | 2E_{final} = kT_{\rm doppler} = \frac{D}{\alpha}
| |
| − | </math>
| |
| − | <math>\alpha</math> is a viscosity parameter: it reflects transport. <math>D</math>
| |
| − | reflects mobility. Thus, this is an Einstein relation, a universal
| |
| − | expression in statistical mechanics resulting from the fundamental
| |
| − | theorem which relates dissipation to fluctuations.
| |
| − | | |
| − | ==== The Doppler limit temperature ====
| |
| − | | |
| − | We've now obtained an expression for the Doppler limit temperature, a
| |
| − | limit on the temperature an ideal two-level atom can be cooled to by
| |
| − | laser beams,
| |
| − | :<math>
| |
| − | k T_{\rm doppler} = \frac{\hbar\Gamma}{2}
| |
| − | \,.
| |
| − | </math>
| |
| − | This optimal temperature is achieved for <math>I\ll I_0</math>, and detuning of
| |
| − | <math>\delta = -\Gamma/2</math> (half a linewidth). Physically, at low
| |
| − | temperatures, the atom cannot determine whether the photon comes from
| |
| − | left or right; at higher temperatures, the atom can discriminate
| |
| − | whether photons come from left or right, thus cooling. For sodium,
| |
| − | this temperature is <math>240</math> <math>\mu</math>K, corresponding to a velocity of <math>30</math>
| |
| − | cm/s.
| |
| − | | |
| − | | |
| − | === 3D molasses, high intensities ===
| |
| − | | |
| − | To cool atoms along not just one axis, but along three axes, use six
| |
| − | counter-propagating laser beams. This configuration is called a 3D
| |
| − | molasses. Everything we've discussed in one dimension can be applied;
| |
| − | just sum up the forces. Some care must be taken, however, if
| |
| − | interference patterns are created between the beams. As long as the
| |
| − | atoms move a distance greater than the wavelength, interference may be
| |
| − | neglected. But large field gradients can add extra forces and
| |
| − | heating.
| |
| − | | |
| − | One can also alternate between the six beams, but having simultaneous
| |
| − | beams actually turns out to be better, due to the interference between the beams. In particular, it gives polarization gradients
| |
| − | and other subtle effects which provide extra cooling. This wasn't initially forseen, but when implemented it was rapidly recognized that 3D cooling with six simultaneous beams was much more powerful than originally thought.
| |
| − | A significant landmark was achieved when, in 1985, Steve Chu used chirped slowing and a 3D molasses
| |
| − | configuration to obtain atoms colder than <math>1</math> mK, for the first time ([https://cua-admin.mit.edu:8443/wiki/images/4/47/Chu85_molasses.pdf Original paper on optical molasses, Chu et al.]).
| |
| − | | |
| − | === Cooling at high intensities ===
| |
| − | | |
| − | Let us consider an example, of laser cooling at high laser intensities. Keep in mind that laser cooling works because <math>F=-\alpha v</math> and
| |
| − | <math>\alpha>0</math>. Assume we have a detuning of about one linewith,
| |
| − | <math>\delta=-\Gamma</math>. Now plot the friction coefficient <math>\alpha</math> as a function of intensity:
| |
| − | | |
| − | ::[[Image:chapter1-intro-to-cooling-Lec1-high-inten.png|thumb|408px|none|]]
| |
| − | Initially, at small intensities, <math>\alpha</math> increases as a function of intensity. Don't be
| |
| − | confused by the fact that the Doppler limit is achieved at low
| |
| − | intensities. The diffusion coefficient is also linear in intensity at
| |
| − | low intensity. <math>\alpha</math> increases with <math>I/I_0</math> at first, and peaks
| |
| − | around <math>0.5</math>, but above the saturation limit <math>\alpha</math> actually changes
| |
| − | sign and starts heating. When <math>\alpha<0</math> then, counter-intuitively,
| |
| − | blue detuned light can be used to cool atoms.
| |
| − | | |
| − | ==== Cooling with blue detuned light ====
| |
| − | | |
| − | This is a
| |
| − | non-trivial result (where does the energy go?), which can be undersood in the
| |
| − | context of the optical Bloch equation and the dressed atom model (see [https://cua-admin.mit.edu:8443/wiki/images/a/af/Ashk83_Earnshaw.pdf Gordon and Ashkin]).
| |
| − | Specifically, the underlying physical reason which allows blue detuned light to cool is that
| |
| − | at low intensities, the force seen by an atom comes from adding to
| |
| − | Lorentzians, as we have seen; this fails at higher intensities, however.
| |
| − | In particular, the optical Bloch equation component <math>u</math> combines with the usual steady state term a new a
| |
| − | velocity dependent term:
| |
| − | :<math>
| |
| − | u = u_{st} + v()
| |
| − | \,.
| |
| − | </math>
| |
| − | One can approximate that <math>u(\vec{r}) \approx
| |
| − | u_{st}(\vec{r}-\vec{v}\Delta t</math>
| |
| − | When you average the light force in the standing wave over an optical
| |
| − | wavelength, then you find an average force which is a friction force,
| |
| − | :<math>
| |
| − | \vec{F} = -\alpha \vec{v}
| |
| − | </math>
| |
| − | At weak intensities, <math>\alpha_{sw} = 2\alpha_{tw}</math>, but at high
| |
| − | intensities <math>\alpha</math> changes sign.
| |
| − | | |
| − | === Momentum and spatial diffusion ===
| |
| − | | |
| − | Let us return to the physics of the randomness of spontaneous light force induced cooling, and revisit the behavior of the diffusion of the cooled atom's momentum and spatial position. We shall see that the physical balance involved is an excellent example of the important fluctuation-dissipation theorem of statistical mechanics.
| |
| − | | |
| − | ==== Momentum diffusion ====
| |
| − | First, consider diffusion of the momentum of an atom being cooled.
| |
| − | The momentum diffusion coefficient is defined as
| |
| − | :<math>\begin{array}{rcl}
| |
| − | 2D^p = \frac{d}{dt} \langle (p- \langle p{\rangle}^2)^2{\rangle}
| |
| − | \,.
| |
| − | \end{array}</math>
| |
| − | This can be directly calculated if we have a fluctuating force, using
| |
| − | the fact that <math>d\vec{p}/dt = \vec{f}</math> is a force:
| |
| − | :<math>\begin{array}{rcl}
| |
| − | 2D^p &=& \frac{d}{dt} \left[
| |
| − | {\langle}\vec{p}\cdot\vec{p}{\rangle}-{\langle}\vec{p}{\rangle}{\langle}\vec{p} \rangle \right]
| |
| − | \\
| |
| − | &=& 2 \left[
| |
| − | {\langle}\vec{p}\cdot\vec{f}{\rangle}-{\langle}\vec{p}{\rangle}{\langle}\vec{f} \rangle \right]
| |
| − | \\ &=& 2 \int^0_{-\infty}
| |
| − | {\langle}\vec{f}(0) \cdot \vec{f}(t) \rangle - \langle \vec{f}(0) {\rangle}{\langle}
| |
| − | \vec{f}(t) \rangle \, dt
| |
| − | \,,
| |
| − | \end{array}</math>
| |
| − | showing that the diffusion is given by the integral of the force-force
| |
| − | correlation function. Essentially:
| |
| − | :<math>\begin{array}{rcl}
| |
| − | 2D^p
| |
| − | &=& 2 \int^0_{-\infty} \langle \vec{f}(t) \cdot \vec{f}(0) \rangle \, dt
| |
| − | \,.
| |
| − | \end{array}</math>
| |
| − | This results due to the fluctuation-dissipation theorem.
| |
| − | | |
| − | === Spatial diffusion ===
| |
| − | This is less frequently discussed in the literature compared with
| |
| − | momentum diffusion, but it is of practical importance in
| |
| − | experiments. Suppose the atoms start in a single point, embedded in a
| |
| − | 3D optical molasses. How does the point distribution expand? On the
| |
| − | time scale determined by <math>\alpha</math>, the atoms loose their memory of
| |
| − | their original velocities. The molasses has a nearly perfect thermal
| |
| − | distribution, despite atoms in the cloud never interacting with each
| |
| − | other, because they thermalize to the laser beam.
| |
| − | | |
| − | The damping time is
| |
| − | :<math>
| |
| − | \frac{1}{\gamma} = \frac{M}{\alpha}
| |
| − | \,.
| |
| − | </math>
| |
| − | Spatial diffusion can be described by a random walk (in space), with a
| |
| − | step size <math>L</math> given by the RMS velocity of the atoms and the damping
| |
| − | time,
| |
| − | :<math>
| |
| − | \ell = 2 \frac{v_{rms}}{\gamma}
| |
| − | \,,
| |
| − | </math>
| |
| − | where the extra factor of <math>2</math> comes from a more rigorous treatment.
| |
| − | Thus, starting from a point distribution, by the standard random walk
| |
| − | result, after time <math>t_d</math>, we obtain
| |
| − | :<math>
| |
| − | \langle r^2 \rangle = 2 \ell^2 t_d \gamma
| |
| − | \,,
| |
| − | </math>
| |
| − | where the number of steps is <math>t_d\gamma</math>. This is
| |
| − | :<math>\begin{array}{rcl}
| |
| − | \langle r^2 \rangle &=& 2 \frac{v_{rms}^2}{\gamma} t_d
| |
| − | \\
| |
| − | \frac{D^p t_d}{\alpha^2}
| |
| − | \,.
| |
| − | \end{array}</math>
| |
| − | Now recall the definition
| |
| − | :<math>
| |
| − | \langle r^2 \rangle = 2 D^x t_d
| |
| − | \,,
| |
| − | </math>
| |
| − | where <math>D^x</math> is the spatial diffusion coefficient.
| |
| − | This gives a relation between the spatial and momentum diffusion
| |
| − | coefficients,
| |
| − | :<math>
| |
| − | D^x = \frac{D^p}{\alpha^2} = \frac{KT}{\alpha}
| |
| − | \,.
| |
| − | </math>
| |
| − | Note the similarity of this expression with the Einstein relation for
| |
| − | carriers in semiconductors, <math>D/\mu = kT/q</math>.
| |
| − | | |
| − | These expressions are useful in the laboratory context, as an example
| |
| − | illustrates. How long does a typical trapped alkali atom (eg cesium
| |
| − | or sodium) take to diffuse out by <math>0.5</math> cm at the Doppler temperature?
| |
| − | Using the formulas above, we get <math>t_d = 1</math> second. This is very
| |
| − | accessible in the laboratory, and is one of the reasons why optical
| |
| − | molasses are so useful in practice.
| |
| | | | |
| | == Beam slowing == | | == Beam slowing == |
Spontaneous emission leads to a redistribution of momentum of an atom, absorbed from incident photons. Here, we discuss three applications of this spontaneous light force:
- Optical Molasses
- Beam Slowing
- Magneto-Optical Traps
Optical Molasses
{{:Optical Molasses}
Beam slowing
We have previously looked at cooling with two laser beams, focusing on
the idea that if you want to cool, you need a force which is linear
with velocity. Now, let us see how you can cool with a single laser
beam. That is what you do when you have a single atomic beam. With a
single laser beam, you can not only slow them down, but also bunch
them up, obtaining the same velocity distribution you get with a
molassas. This is the only example of laser cooling I know of which
has a very simple, closed form solution, which is possible because
there is only one laser beam.
Imagine, if you have a single Lorentzian, how can you cool?
Generically, you need a ``lock point, which is stable and has atoms
moving to that point in phase space from either velocity direction.
With a single beam you don't apparently have such, but as we shall see
there is something else interesting that can be done.
Consider a single beam of atoms, with this velocity distribution.
Using a laser, you can push some of the atoms to become cooler (red
line below), bunching them up in a lower velocity regime, and leaving
atoms faster than some velocity unchanged. What do you have to do if
you want to bunch all the atoms at zero velocity? It becomes clear
that you want more than one laser frequency, for example. By applying
a broad range of frequencies which cover the whole velocity
distribution, you can slow down all the atoms. There are several
techniques using such an approach, known as white light slowing, or
diffuse light slowing. But none of those are as powerful as
{\em chirpedd slowing} and {\em Zeeman slowing}, the techniques of choice
in modener laser cooling.
Chirped slowing
The idea behind chirped slowing is to get the atoms to "ride the surf". In other words, the frequency chirp of the laser beam and the deceleration of the atoms should be synchronized.
Balance of equations
Step 1
The force on an atom in the beam due to the light is
![{\displaystyle F=-\hbar k{\frac {\Gamma }{2}}{\frac {I/I_{0}}{1+I/I_{0}+\left[{\frac {2(\delta +kv)}{\Gamma }}\right]^{2}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5dfb969d3cde2f98dcdf4cf800b63c3543426809)
Let  denote the atom's acceleration.
Let us assume a frame of reference and experimental setup such that
denote the atom's acceleration.
Let us assume a frame of reference and experimental setup such that
 ,
,  ,
,  ,
,  . We can call
. We can call  , where
, where  is the atom's mass.
is the atom's mass.
Step 2
The scheme begins by selecting the deceleration desired, some  .
Then set
.
Then set  , and look for a
, and look for a  to obtain
this desired force.
This will exist if
to obtain
this desired force.
This will exist if

Step 3
Next, select an initial velocity  such that
such that  .
.
 is the detuning for this "targeted" velocity group, so we
must provide a laser with frequency in the lab frame of
is the detuning for this "targeted" velocity group, so we
must provide a laser with frequency in the lab frame of  . The atom's velocity will differ from the desired
target group by
. The atom's velocity will differ from the desired
target group by  .
.
Step 4
With these definitions, we now have
![{\displaystyle F=-\hbar k{\frac {\Gamma }{2}}{\frac {I/I_{0}}{1+I/I_{0}+\left[{\frac {2(\delta '+kv')}{\Gamma }}\right]^{2}}}\,,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/087622d4991f57f7abd12a596702081e4efe6480)
in the frame of reference of the atoms in the target velocity group.
Step 5
Transforming into this decelerating frame, we get a fictitious force
with is  , and
, and
![{\displaystyle F'(v')=Ma_{max}\left[{\frac {I/I_{0}}{1+I/I_{0}+\left[{\frac {2(\delta '+kv')}{\Gamma }}\right]^{2}}}+{\frac {I/I_{0}}{1+I/I_{0}+\left[{\frac {2(\delta ')}{\Gamma }}\right]^{2}}}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a0772bab6a738e2ee29b5c0ea76102175dfe6e28)
This second term has the same structure as the first, but it is
velocity independent. Note that this is exact, and valid for arbitrary  . All we've done is to substitute definitions, so far, but they provide useful intuition.
. All we've done is to substitute definitions, so far, but they provide useful intuition.
Note that for small  , this force is linear in velocity,
, this force is linear in velocity,  .
.
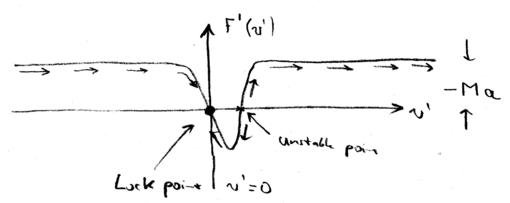
Decelerating Frame
In the lab frame, we have a force which is a positive Lorentzian. In
the decelerating frame, we had to add  (the inertial force),
so this Lorentzian shifts down, when we plot the total force as a function of the velocity in the decelerating frame
(the inertial force),
so this Lorentzian shifts down, when we plot the total force as a function of the velocity in the decelerating frame  :
:
Therefore, there is now a stable "lock" point, where  as a
function of
as a
function of  . In other words, when the atoms have a negative velocity
. In other words, when the atoms have a negative velocity  , the atoms all accelerate towards zero velocity (in the decelerating frame). And when atoms have a small positive velocity, they also move towards
, the atoms all accelerate towards zero velocity (in the decelerating frame). And when atoms have a small positive velocity, they also move towards  . In contrast the point where the Lorentzian has
. In contrast the point where the Lorentzian has  at
at  , is unstable.
, is unstable.
Thus, we may write, as we did with the molasses, an
expression for the linearized force around this point,  , in which
, in which  . There is also randomness, as there was in the optical molassas case, for which we may calculate a momentum diffusion
coefficient, and we find that
. There is also randomness, as there was in the optical molassas case, for which we may calculate a momentum diffusion
coefficient, and we find that  . Thus,
the final temperature limit of the beam is actually the same as that
achievable with a molasses:
. Thus,
the final temperature limit of the beam is actually the same as that
achievable with a molasses:  .
.
We've seen that one laser can bunch up atoms from a beam at a single
velocity. Physically, what happens is that if the atoms fall behind,
the light does not interact with them, but if the atoms are too fast,
the laser cools them, much like in the molasses case.
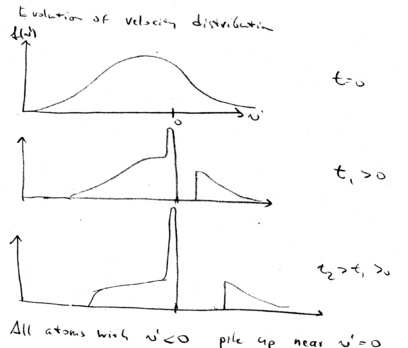
Graphical summary
Here is a graphical summary of what we've learned about beam slowing.
In the decelerating frame, this is the situation. Change sign, so
that in the frame the decelerating force is positive, for this graph:
Initially, our zero force point is at the targeted velocity  .
All atoms at larger velocity experience a constant positive force,
accelerating them. After a later time
.
All atoms at larger velocity experience a constant positive force,
accelerating them. After a later time  , the tail of the
maxwell-Boltzmann distribution is pushed to higher velocities. The peak of distribution of atoms grows higher and higher with time, while the positive tail moves to higher velocities.
, the tail of the
maxwell-Boltzmann distribution is pushed to higher velocities. The peak of distribution of atoms grows higher and higher with time, while the positive tail moves to higher velocities.
This is the description in the decelerating frame.
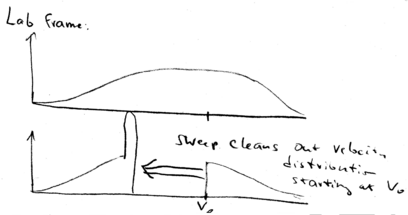
In the lab frame, we shift from  back to
back to  . This means that we just shift all the distributions down in velocity. We start with a broad distribution at
. This means that we just shift all the distributions down in velocity. We start with a broad distribution at  . There is a velocity group at
. There is a velocity group at  which is on resonance with the laser beam. As time goes on, the laser beam chirps, causing more and more atoms to come into resonance, piling them up in a lower velocity class. What happens is that after the laser beam is switched off, you may still have a low velocity tail which is not reached by the laser, but you have a huge peak of atoms that have been cooled:
which is on resonance with the laser beam. As time goes on, the laser beam chirps, causing more and more atoms to come into resonance, piling them up in a lower velocity class. What happens is that after the laser beam is switched off, you may still have a low velocity tail which is not reached by the laser, but you have a huge peak of atoms that have been cooled:
All the atoms at lower velocities are pushed up in velocity
until they stack up at  , producing a narrow distribution around
, producing a narrow distribution around
 .
.
The width of this narrow velocity distribution is given by  , which is proportional to
, which is proportional to
 .
.
Beam cooling is actually the simplest and cleanest example of laser
cooling. It has the same physics as the molasses case, with the second beam being replaced in a sense by a ficticious force. Note that in the two-beam molasses case, there are more complications, since one should really
consider interference effects.
Energy conservation in laser cooling
Kinetic energy conservation
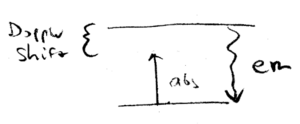
Where does the lost kinetic energy go, in cooling the atoms? In the beam cooling scenario, for example, you have moved a large number of atoms from high velocities to low velocities. How can this be reconciled with energy conservation? Well, there are only several possibilities for where the energy can go. In particular, there is light scattered by the atoms, and the energy is radiated away by spontaneous emission, as we shall now see.
Absorption is responsible for momentum change, and emission is responsible for energy conservation. Light emitted by the atom is at the resonant energy  , but
can be absorbed when the photon is just slightly less than
, but
can be absorbed when the photon is just slightly less than  .
The emission is isotropic, whereas the incident light is directed and
Doppler shift dependent.
.
The emission is isotropic, whereas the incident light is directed and
Doppler shift dependent.
Doppler cooling can be explained in this picture. Incident laser light is
detuned below  ("red detuned"), whereas emitted photons are, on average, at a higher frequency, so more energy is emitted than is absorbed, when an atom is cooled.
("red detuned"), whereas emitted photons are, on average, at a higher frequency, so more energy is emitted than is absorbed, when an atom is cooled.
Energy conservation in general cooling schemes
The same intuition can be applied to all cooling schemes, including those used to cool solids and liquids. Phonon
assisted absorption is balanced against emission, resulting in cooling:
How hard is it to cool liquids and solids? Consider a system at
 K; that gives the phonon energy. Then
K; that gives the phonon energy. Then  Kelvin. In practice, there is a lower than unity fluorescence quantum
yield, because there are non-radiative ways to exit the excited
state. The cooling will be efficient, however, only when the quantum
yield is higher than
Kelvin. In practice, there is a lower than unity fluorescence quantum
yield, because there are non-radiative ways to exit the excited
state. The cooling will be efficient, however, only when the quantum
yield is higher than  , which is typically unrealistic.
Cooling with laser light is therefore not typically practical, for
systems other than atoms, which have a unity fluorescence quantum
yield. Molecules are hard, because they have non-radiative
de-excitation pathways.
, which is typically unrealistic.
Cooling with laser light is therefore not typically practical, for
systems other than atoms, which have a unity fluorescence quantum
yield. Molecules are hard, because they have non-radiative
de-excitation pathways.
References
![{\displaystyle F=-\hbar k{\frac {\Gamma }{2}}{\frac {I/I_{0}}{1+I/I_{0}+\left[{\frac {2(\delta +kv)}{\Gamma }}\right]^{2}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5dfb969d3cde2f98dcdf4cf800b63c3543426809)















![{\displaystyle F=-\hbar k{\frac {\Gamma }{2}}{\frac {I/I_{0}}{1+I/I_{0}+\left[{\frac {2(\delta '+kv')}{\Gamma }}\right]^{2}}}\,,}](https://wikimedia.org/api/rest_v1/media/math/render/svg/087622d4991f57f7abd12a596702081e4efe6480)

![{\displaystyle F'(v')=Ma_{max}\left[{\frac {I/I_{0}}{1+I/I_{0}+\left[{\frac {2(\delta '+kv')}{\Gamma }}\right]^{2}}}+{\frac {I/I_{0}}{1+I/I_{0}+\left[{\frac {2(\delta ')}{\Gamma }}\right]^{2}}}\right]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a0772bab6a738e2ee29b5c0ea76102175dfe6e28)