Difference between revisions of "Van der Waals interaction"
imported>Ichuang |
imported>Ichuang |
||
| Line 206: | Line 206: | ||
Consider three scenarios: | Consider three scenarios: | ||
| − | * | + | * Atom-atom. At long distances <math>V\propto |
| − | \frac{\alpha_1\alpha_2}{R^7}</math> | + | \frac{\alpha_1\alpha_2}{R^7}</math>, but at short distances <math>V\propto\frac{1}{R^6}</math> |
| − | * | + | * Atom-wall. Recall that the polarizibility of an atom has units of volume, <math>R^3</math>. A large metal sphere also has polarizability <math>R^3</math>. Thus, at long distances, <math>V\propto\frac{\alpha R^3}{R^7} \sim\frac{\alpha}{R^4}</math>. |
| − | Recall that the polarizibility of an atom has units of volume, <math>R^3</math>. | + | ::[[Image:vdw-fig4.png|thumb|306px|none|]] |
| − | A large metal sphere also has polarizability <math>R^3</math>. Thus, at long | + | * Two walls. Two walls can be regarded as two spheres, with a radius similar to their distance. The potential is now 1/R, but we have to normalize it to the area <math>\frac{V}{A}\propto \frac{1}{R R^2} \sim \frac{1}{R^3}</math>. |
| − | distances, <math>V\propto\frac{\alpha R^3}{R^7} \sim\frac{\alpha}{R^4}</math>. | + | ::[[Image:vdw-fig5.png|thumb|406px|none|]] |
| − | * | ||
| − | Two walls can be regarded as two spheres, with a radius similar to their distance. The potential is now 1/R, but we have to normalize it to the area | ||
| − | <math>\frac{V}{A}\propto \frac{1}{R R^2} \sim | ||
| − | |||
At short distances: | At short distances: | ||
Revision as of 13:56, 21 April 2009
Today - the Van der Waals interaction between two atoms.
Contents
Two physical pictures of the van der Waals interaction: classical and quantum
Two physical pictures illuminate the richness of the problem. Consider two atoms and separated by distance . The Coulomb interaction between these is
Integrating leaves us with two interacting dipoles
In the quantum picture, these dipoles become operators. First order perturbation theory tells us that if one atom is excited and the other is in the ground state, this gives an interaction term
But if both atoms are in the ground state, then the leading term is given by second order perturbation theory, as
Physical interpretation
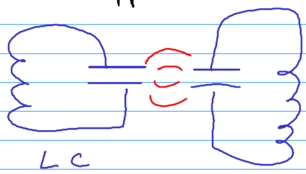
Let us interpret this result now (see Kleppner's class notes) - the physics of the van der Waals interaction are all due to quantum fluctuations. Consider two resonant circuits that are capactively coupled:
The coupling goes as . Each resonator has frequency , and classically coupled oscillators split their frequencies, giving
The ground state energy is . The two add up to zero leaving just the term.
An equivalent description focusing on the atomic dipole moment is as follows. A neutral atom has no expectation for the dipole operator, , but the variance for this is nonzero, . This is just as the simple harmonic oscillator in its ground state has zero , but nonzero in the ground state. The fluctuating dipole moment of atom creates a fluctuating field at position ,
This induces a dipole in atom . The interaction energy between two dipoles is
Origin in fluctuations of the electromagnetic field
So far, we've neglected fluctuations of the electromagnetic field. What could the vacuum fluctuations of the field do to neutral atoms? Fluctuations at position and can induce dipole moments, since the atoms are polarizable:
The corresponding interaction is
See Larry Spuch, Physics Today article, posted on web site for more about this. Conceptually, one sums over all modes of the electromagnetic field - this time in free space, without the boundary condition as we do in the Casimir force case. The long wavelengths do not average out to zero, when they are of wavelength comparable to the distance between the two atoms. Their contribution, when density of states are included, and cutoff appropriately, gives
So how does the term disappear? If you focus on the fluctuations of the instantaneous dipole moments, then you get ; this is the correct result for short range interactions. It can be derived, as we showed above, by conceptually using the uncertainty principle for the atomic dipoles. In contrast, when the uncertainty principle for the electromagnetic field is used, we get a force; this is applicable at long ranges, and is known as the Casimir-Polder effect, or the retarded van der Waals interaction. Note that a full quantum theory of both atoms and field are needed to get both of these interactions properly. The crossover distance between the two interactions is set by , the resonant wavelength of that atoms.
The entirety of the van der Waals interaction is a quantum phenomenon (thus, the reason your table is solid is because of quantum mechanics!). Classically, there is no average value of the dipole moment, and also no fluctuations - the classical van der Waals force is zero. But quantum-mechanically, vacuum fluctuations are always present. It doesn't cost extra energy to create them. For the van der Waals force, the dipole fluctuations simply become correlated and lower the total energy of the system (by leading to an attractive interaction energy).
Van der Waals forces due to absorption and emission of virtual photons
Returning to our theme of perturbation theory for this chapter, let us consider the van der Waals interaction from a diagrammatic approach. (This follows API pp 121-126). The fundamental idea is an exchange of virtual photons. The Hamiltonian in the dipole approximation is
In deriving the QED Hamiltonian, we discarded the longidutional fields because they were determined by the charges and not a degree of freedom, and we kept the transverse fields, as the radiation field. In the qualitative discussion above, we obtained the dipole moments and their interactions from a Coulomb integral. However, in our QED approach, outside the neutral atoms there only exists the radiation field. We want to understand now how this can result in the van der Waals interaction.
The only way two neutral atoms can interact with each other is by exchange of virtual photons. See Cohen-Tanoudji for hundreds of pages of illuminative discussion about this.
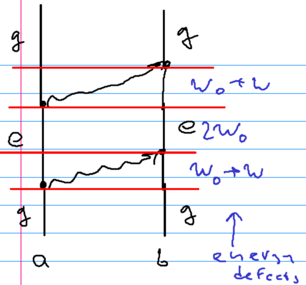
The language of Feynman diagrams can be used to understand the van der Waals interaction. Consider two identical atoms spaced apart by distance . The atoms initially start in state , the ground state. One emits a virtual photon, going into a virtual excited state (with an energy below the ground state). This photon is absorbed by the second atom. After a short time, the first atom emits another photon, which is absorbed by the second one, returning both atoms to the ground state:
This picture indicates that to leading order, we need diagrams with two photons, and four vertices. The dominant contribution comes from photons with , that is . Another important piece of guidance is that the virtual process is admixing states of higher energy to the ground state, lowering the total energy; energy shifts in second order processes go as
characterizing the smaller amplitude of mixing with higher energy states. With extra intermediate states this becomes
When we emit virtual photons, there are two energies involved; one is the atomic excitation energy , the other one is the exchanged photon energy . The energy defects in the above picture are thus as shown in the figure above: initially , then , then .
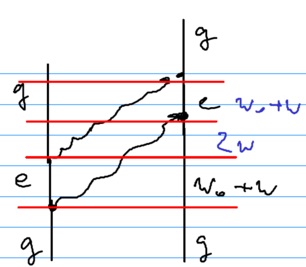
A second diagram involved is as if one atom emitted two photons simultaneously:
Which of these two diagrams is preferable depends on how large is compared to . Ultimately, that distinguishes short and long range interactions, and versus . The energy defects in the three regions are , then (versus for the first diagram), and then .
The contribution of each diagram is proportional to the inverse product of the energy defects:
In contrast, for the second diagram, we get
Focusing on these two diagrams, let us consider what happens at short and long ranges. Let be the wavelength of the radiation. Short range is defined by (meaning ), and long range is (meaning ). Thus for long range, the first diagram dominates, whereas for short range, the second. Thus, going from long range to short range involves an additional factor of in the interaction. Since , this gives the change from to in the potential.
Casimir forces revisted
Let us now look at Casimir forces for different systems.
Consider three scenarios:
- Atom-atom. At long distances , but at short distances
- Atom-wall. Recall that the polarizibility of an atom has units of volume, . A large metal sphere also has polarizability . Thus, at long distances, .
- Two walls. Two walls can be regarded as two spheres, with a radius similar to their distance. The potential is now 1/R, but we have to normalize it to the area .
At short distances:
Both the atom-atom and the atom-wall interaction change in the power law by one factor of R, whereas the wall-wall interaction is always in the far field (A metallic wall corresponds to an atom with high excitation energy and therefore small wavelength).
- atom-atom.
- atom-wall.
- two walls. Always in the far field.