Difference between revisions of "Atoms in Magnetic Fields"
imported>Ketterle |
imported>Idimitro |
||
| (31 intermediate revisions by 7 users not shown) | |||
| Line 42: | Line 42: | ||
{\bf{L}} | {\bf{L}} | ||
</math> | </math> | ||
| − | where <math>\gamma_{\ell}</math> is referred to as the | + | where <math>\gamma_{\ell}</math> is referred to as the ''gyromagnetic ratio''. This is a general result for any turbulently rotating blob |
| − | ratio | ||
provided only that it has a constant ratio of charge to mass | provided only that it has a constant ratio of charge to mass | ||
throughout. | throughout. | ||
| Line 51: | Line 50: | ||
{\bf{L}} /\hbar | {\bf{L}} /\hbar | ||
</math> | </math> | ||
| − | which is the classical result, and <math>\mu_B</math> is the | + | which is the classical result, and <math>\mu_B</math> is the ''Bohr magneton'': |
| − | magneton | ||
:<math> | :<math> | ||
| − | \mu_B = \frac{e\hbar}{2m} = | + | \mu_B = \frac{e\hbar}{2m} = 9.27408(4)\times 10^{-24} J/T |
| − | \rightarrow | + | \rightarrow 1.39983 \times 10^4 MHz \times B/T |
</math> | </math> | ||
=== Intrinsic electron spin and magnetic moment === | === Intrinsic electron spin and magnetic moment === | ||
| − | When Uhlenbeck and Goudsmit suggested | + | When Uhlenbeck and Goudsmit suggested <ref> G. E. Uhlenbeck and S. Goudsmit. Spinning electrons and the structure of spectra. Nature, 117(2938):264, Feb 1926. </ref> that the |
electron had an intrinsic spin <math>S = \frac{1}{2}</math>, it soon became | electron had an intrinsic spin <math>S = \frac{1}{2}</math>, it soon became | ||
apparent that it had a magnetic moment twice as large as would be | apparent that it had a magnetic moment twice as large as would be | ||
| Line 117: | Line 115: | ||
:<math>\begin{align} | :<math>\begin{align} | ||
g_j &= \frac{ {\bf{L}} \cdot ( {\bf{L}} + { {\bf{S}} })+ 2 { {\bf{S}} } \cdot | g_j &= \frac{ {\bf{L}} \cdot ( {\bf{L}} + { {\bf{S}} })+ 2 { {\bf{S}} } \cdot | ||
| − | ( {\bf{L}} +{ {\bf{S}} })}{ \left| {{\bf{J}}} \right| | + | ( {\bf{L}} +{ {\bf{S}} })}{ \left| {{\bf{J}}} \right|^2} \\ |
| − | &= 1 + \frac{j(j+1) + s(s+1) - \ell (\ell + 1)}{ | + | &= 1 + \frac{j(j+1) + s(s+1) - \ell (\ell + 1)}{2j(j+1) } |
\end{align}</math> | \end{align}</math> | ||
using <math>2 {\bf{L}} \cdot { {\bf{S}} } = { {\bf{J}} }^2 - {\bf{L}} ^2 - { {\bf{S}} }^2</math>. | using <math>2 {\bf{L}} \cdot { {\bf{S}} } = { {\bf{J}} }^2 - {\bf{L}} ^2 - { {\bf{S}} }^2</math>. | ||
| Line 144: | Line 142: | ||
== Hyperfine structure in an applied field == | == Hyperfine structure in an applied field == | ||
| − | Now we add the nuclear angular momentum to the picture. The Hamiltonian in an applied field <math> { {\bf{B}} }_0 </math> is | + | Now we add the nuclear angular momentum to the picture. The Zeeman Hamiltonian in an applied field <math> { {\bf{B}} }_0 </math> is |
| + | :{EQ_hsaf1} | ||
:<math> | :<math> | ||
| − | + | H_z= a h { {\bf{I}} } \cdot { {\bf{J}} } - {\bf{\mu}} _J \cdot { {\bf{B}} }_0 - | |
| − | {\bf{\mu}} _I \cdot { {\bf{B}} }_0 = a h { {\bf{I}} } \cdot { {\bf{J}} } + g_j \mu_B { {\bf{J}} } \cdot { {\bf{B}} }_0 - g_I \mu_B | + | {\bf{\mu}} _I \cdot { {\bf{B}} }_0 = a h { {\bf{I}} } \cdot { {\bf{J}} } + g_j \mu_B { {\bf{J}} } \cdot { {\bf{B}} }_0 - g_I^\prime \mu_B |
{ {\bf{I}} } \cdot { {\bf{B}} }_0 | { {\bf{I}} } \cdot { {\bf{B}} }_0 | ||
</math> | </math> | ||
By convention, we take <math> {\bf{\mu}} _J = - g_j\mu_B { {\bf{J}} }</math>. Note | By convention, we take <math> {\bf{\mu}} _J = - g_j\mu_B { {\bf{J}} }</math>. Note | ||
that we are expressing the nuclear | that we are expressing the nuclear | ||
| − | moment in terms of the Bohr magneton, and that <math>g_I << g_j</math>. (The | + | moment in terms of the Bohr magneton, and that <math>g_I^\prime << g_j</math>. (The |
nuclear moment is often expressed | nuclear moment is often expressed | ||
in terms of the nuclear magneton, in which case <math> {\bf{\mu}} _I </math> = | in terms of the nuclear magneton, in which case <math> {\bf{\mu}} _I </math> = | ||
| − | <math> | + | <math>g_I\mu_N { {\bf{I}} }</math>, where |
| − | <math>\mu_N</math> is the nuclear magneton.) What are the quantum numbers and | + | <math>\mu_N</math> is the nuclear magneton.) |
| + | |||
| + | What are the quantum numbers and | ||
energies? Before discussing the | energies? Before discussing the | ||
general solution, let us look at the limiting cases. | general solution, let us look at the limiting cases. | ||
| + | |||
=== Low field === | === Low field === | ||
The total angular momentum is <math> { {\bf{F}} }= { {\bf{I}} }+ { {\bf{J}} }</math>. In low | The total angular momentum is <math> { {\bf{F}} }= { {\bf{I}} }+ { {\bf{J}} }</math>. In low | ||
| Line 175: | Line 177: | ||
\langle{{ {\bf{J}} }\cdot { {\bf{B}} }_0 }\rangle = | \langle{{ {\bf{J}} }\cdot { {\bf{B}} }_0 }\rangle = | ||
| − | \frac{\langle{{ {\bf{J}} } | + | \frac{\langle{{ {\bf{J}} }\cdot { {\bf{F}} }}\rangle \langle{ {\bf{F}} }\cdot { {\bf{B}} }_0 \rangle } {{ \left| {{\bf{F}}} \right|} ^2} |
</math> | </math> | ||
:<math> | :<math> | ||
| − | H_z = - \mu_B {\left[ -g_j ({ {\bf{J}} }\cdot { {\bf{F}} }) + g_I ({ {\bf{I}} }\cdot { {\bf{F}} })\right]} \frac{{ {\bf{F}} }\cdot { {\bf{B}} }_0}{F^2} | + | H_z = - \mu_B {\left[ -g_j ({ {\bf{J}} }\cdot { {\bf{F}} }) + g_I ({ {\bf{I}} }\cdot { {\bf{F}} })\right]} \frac{{ {\bf{F}} }\cdot { {\bf{B}} }_0} {{ \left| {{\bf{F}}} \right|} ^2} |
</math> | </math> | ||
| − | Since <math> g_I \ll g_j </math>, we can usually neglect it. We can rewrite | + | Since <math> g_I^\prime \ll g_j </math>, we can usually neglect it. We can rewrite |
this result as | this result as | ||
:<math> | :<math> | ||
| Line 189: | Line 191: | ||
:<math> | :<math> | ||
| − | g_F = \frac{ \langle{{ {\bf{J}} }\cdot { {\bf{F}} }}\rangle}{F^2} g_j = \frac{g_j}{2} | + | g_F = \frac{ \langle{{ {\bf{J}} }\cdot { {\bf{F}} }}\rangle} {{ \left| {{\bf{F}}} \right|} ^2} g_j = \frac{g_j}{2} |
\frac{F(F+1) + j (j+1) - I | \frac{F(F+1) + j (j+1) - I | ||
(I+1)}{F(F+1)} | (I+1)}{F(F+1)} | ||
</math> | </math> | ||
| − | + | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
::[[Image:Atoms_in_Magnetic_Fields-B.png|thumb|400px|none|]] | ::[[Image:Atoms_in_Magnetic_Fields-B.png|thumb|400px|none|]] | ||
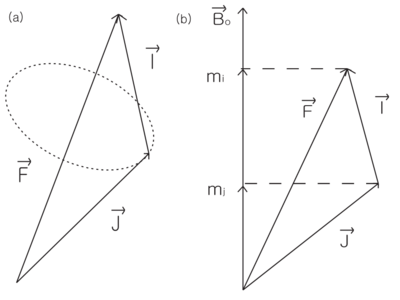
| − | + | Total angular momentum F=I+J. (a) In low field, only the components of | |
J and I parallel to F are important. (b) In high field, <math>m_i</math> and <math>m_j</math> are good | J and I parallel to F are important. (b) In high field, <math>m_i</math> and <math>m_j</math> are good | ||
| − | quantum numbers. | + | quantum numbers. |
| − | + | ||
| − | |||
=== High field === | === High field === | ||
| − | If <math> {\bf{\mu}} _j\cdot { {\bf{B}} }_0 \gg a h { {\bf{I}} }\cdot { {\bf{J}} }</math>, then | + | If <math> {\bf{\mu}} _j\cdot { {\bf{B}} }_0 \gg a h { {\bf{I}} }\cdot { {\bf{J}} }</math>, then <math>{\bf{J}}</math> is quantized along |
| − | |||
<math>{ {\bf{B}} }_0</math>. Although <math> {\bf{\mu}} _I\cdot { {\bf{B}} }_0 </math> is not | <math>{ {\bf{B}} }_0</math>. Although <math> {\bf{\mu}} _I\cdot { {\bf{B}} }_0 </math> is not | ||
necessarily large compared to the | necessarily large compared to the | ||
hyperfine interaction, the <math> { {\bf{I}} } \cdot { {\bf{J}} }</math> coupling | hyperfine interaction, the <math> { {\bf{I}} } \cdot { {\bf{J}} }</math> coupling | ||
| − | assures that | + | assures that <math>{\bf{I}}</math> is also quantized |
along <math>{ {\bf{B}} }_0</math>. Thus <math>m_I</math> and <math>m_j</math> are good quantum numbers. | along <math>{ {\bf{B}} }_0</math>. Thus <math>m_I</math> and <math>m_j</math> are good quantum numbers. | ||
| − | In this case, | + | In this case, the Zeeman Hamiltonian has the from |
:<math> | :<math> | ||
| − | + | H_z=ahm_im_j + g_j\mu_B m_j B_0 - g_I^\prime \mu_B m_I B_0 | |
| − | |||
</math> | </math> | ||
The second term on the right is largest. Usually the first term is | The second term on the right is largest. Usually the first term is | ||
next largest, and the nuclear | next largest, and the nuclear | ||
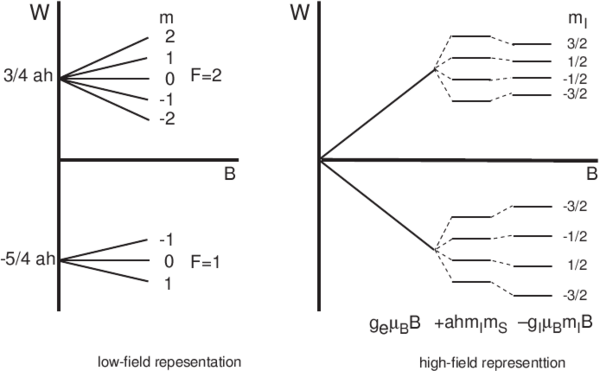
| − | terms is smallest. Figure | + | terms is smallest. The Figure below shows low and high field |
behavior for hyperfine structure for <math>I=3/2, J = 1/2 </math>. | behavior for hyperfine structure for <math>I=3/2, J = 1/2 </math>. | ||
| − | + | ||
| − | + | ::[[Image:Atoms_in_Magnetic_Fields-B-field-levels.png|thumb|600px|none|]] | |
| − | ::[[Image:Atoms_in_Magnetic_Fields-B-field-levels.png|thumb| | + | Energy level structure for a single-electron atom with nuclear spin 3/2 |
| − | + | in the limits of low and high fields. | |
| − | in the limits of low and high fields. | + | |
| − | |||
| − | |||
=== General solution === | === General solution === | ||
| Line 242: | Line 231: | ||
and high field, bearing in mind | and high field, bearing in mind | ||
that <math> m= m_I + m_j</math> is a good quantum number at all fields. | that <math> m= m_I + m_j</math> is a good quantum number at all fields. | ||
| − | For <math>J = 1/2</math>, the eigenvalues of | + | For <math>J = 1/2</math>, the eigenvalues of Eq. \ref{EQ_hsaf1} can be |
| − | found exactly. The energies are | + | found exactly (see homework). The energies are |
given by the Breit-Rabi formula | given by the Breit-Rabi formula | ||
:<math> | :<math> | ||
| Line 266: | Line 255: | ||
Physically, <math>x</math> is the ratio of the paramagnetic interaction (the "Zeeman | Physically, <math>x</math> is the ratio of the paramagnetic interaction (the "Zeeman | ||
energy") to the hyperfine separation. The Breit-Rabi energy level diagram for | energy") to the hyperfine separation. The Breit-Rabi energy level diagram for | ||
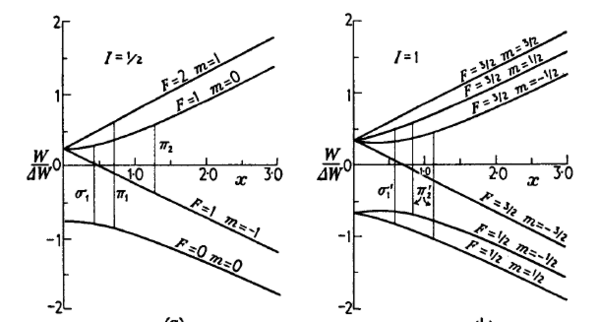
| − | hydrogen and deuterium are shown in figure | + | hydrogen and deuterium are shown in the figure. The units |
reflect current interest in atom trapping. Low-field quantum numbers are shown. | reflect current interest in atom trapping. Low-field quantum numbers are shown. | ||
It is left as an exercise to identify the high field quantum numbers. | It is left as an exercise to identify the high field quantum numbers. | ||
| − | + | ||
| − | + | ::[[Image:Atoms_in_Magnetic_Fields-Breit-Rabi.png|thumb|600px|none|]] | |
| − | ::[[Image:Atoms_in_Magnetic_Fields-Breit-Rabi.png|thumb| | + | Energy level structure for a single-electron atom with nuclear spin I |
| − | + | = 1/2, such as hydrogen (left), and I = 1, such as deuterium (right). Note the typo on the left: The top state is F=1 not F=2. From | |
| − | = 1/2, such as hydrogen (left), and I = 1, such as deuterium (right). From | + | ''Molecular Beams'' by N.F. Ramsey <ref> Norman F. Ramsey. Molecular Beams. Clarendon Press, 1956. </ref>. |
| − | Molecular Beams | + | |
| + | == References == | ||
| + | <ul><li> '''Lande1923''': A. Landé. ''Zeitschrift für Physik'', 15:189, 1923. | ||
| + | <li> '''Ramsey1956''': Norman F. Ramsey. ''Molecular Beams''. Clarendon Press, 1956. | ||
| + | <li> '''Uhlenbeck1926''': G. E. Uhlenbeck and S. Goudsmit. Spinning electrons and the structure of spectra. ''Nature'', 117(2938):264, Feb 1926. | ||
| + | </ul> | ||
| + | <br style="clear: both" /> | ||
== Notes == | == Notes == | ||
[[Category:8.421]] | [[Category:8.421]] | ||
Latest revision as of 18:36, 18 October 2015
Contents
Fine structure in applied magnetic fields
In this section we treat the interaction of the electron's orbital and spin angular momentum with external static magnetic fields. Previously, in the chapter on fine structure, we have considered the spin-orbit interaction: the coupling of electron spin to the magnetic field generated by the nucleus (which appears to move about the electron in the electron's rest frame). The spin orbit interaction causes the orbital and spin angular momenta of the electron to couple together to produce a total spin which then couples to the external field; the magnitude of this coupling is calculated here for weak external fields.
We first discuss the magnetic moment due to orbital angular momentum and spin angular momentum, and then we put things together.
Magnetic moment of circulating charge (classical)
The energy of interaction of a classical magnetic moment with a magnetic field is
indicating that the torque tends to align the moment along the field. In classical electrodynamics the magnetic moment of a moving point particle about some point in space is independent of the path which it takes, but depends only on the product of the ratio of its charge to mass , and angular momentum . This result follows from the definitions of angular momentum
and magnetic moment
where is the current and the velocity (see Jackson Ch.5). The equality of the bracketed terms implies
where is referred to as the gyromagnetic ratio. This is a general result for any turbulently rotating blob provided only that it has a constant ratio of charge to mass throughout. For an electron with orbital angular momentum
which is the classical result, and is the Bohr magneton:
Intrinsic electron spin and magnetic moment
When Uhlenbeck and Goudsmit suggested [1] that the electron had an intrinsic spin , it soon became apparent that it had a magnetic moment twice as large as would be expected on the basis of the treatment above. (This implies that the electron cannot be made out of material with a uniform ratio of charge to mass.) This is accounted for by writing for the intrinsic electron moment
where the quantity is called the electron -factor. (The negative sign permits treating as a positive quantity, which is the convention.) This factor was predicted by the Dirac theory of the electron, probably its greatest triumph. Later, experiments by Kusch, followed by Crane et al., and then by Dehmelt and coworkers, have shown (for both electrons and positrons).
This result has been calculated from quantum electrodynamics, which gives
The agreement betwen the prediction of quantum electrodynamics and experiment on the electron g-factor is often cited as the most precise test of theory in all of physics.
The Lande g-factor
In zero or weak magnetic field, the Hamiltonian is
Since the second term (LS coupling) corresponds to an internal B field of about one Tesla, we will consider the limit of a weak external magnetic field.
The spin orbit interaction couples and together to form . For the Zeeman Hamiltonian, we therefore have to project all angular momenta and the magnetic field along the direction of :
We want to write this as
which defines the so-called Lande g factor as
Taking
using .
If a transition from a level with angular momentum to a level with takes place in a magnetic field, the resulting spectral line will be split into three or more components---a phenomenon known as the Zeeman effect. For transitions with a particular , say , the components will have shifts
If (or if or ) then will not depend on (or there will be only one transition with ) and there will be only 3 components of the line (); this is called the normal Zeeman splitting. If neither of these conditions holds, the line will be split into more than 3 components and the Zeeman structure is termed "anomalous"---it can't be explained with classical atomic models.
Hyperfine structure in an applied field
Now we add the nuclear angular momentum to the picture. The Zeeman Hamiltonian in an applied field is
- {EQ_hsaf1}
By convention, we take . Note that we are expressing the nuclear moment in terms of the Bohr magneton, and that . (The nuclear moment is often expressed in terms of the nuclear magneton, in which case = , where is the nuclear magneton.)
What are the quantum numbers and energies? Before discussing the general solution, let us look at the limiting cases.
Low field
The total angular momentum is . In low field, and are good quantum numbers. Each level contains degenerate states. In a weak field the () fold degeneracy is lifted. We can treat the terms
as a perturbation. and are not good quantum numbers, only their components parallel to are important. Thus
Since , we can usually neglect it. We can rewrite this result as
Total angular momentum F=I+J. (a) In low field, only the components of J and I parallel to F are important. (b) In high field, and are good quantum numbers.
High field
If , then is quantized along . Although is not necessarily large compared to the hyperfine interaction, the coupling assures that is also quantized along . Thus and are good quantum numbers. In this case, the Zeeman Hamiltonian has the from
The second term on the right is largest. Usually the first term is next largest, and the nuclear terms is smallest. The Figure below shows low and high field behavior for hyperfine structure for .
Energy level structure for a single-electron atom with nuclear spin 3/2 in the limits of low and high fields.
General solution
Finding eigenfunctions and eigenvalues of the hyperfine Hamiltonian for arbitrary field requires diagonalizing the energy matrix in some suitable representation. To obtain a rough idea of the expected results, one can smoothly connect the energy levels at low and high field, bearing in mind that is a good quantum number at all fields. For , the eigenvalues of Eq. \ref{EQ_hsaf1} can be found exactly (see homework). The energies are given by the Breit-Rabi formula
where the sign is for , and the sign is for . is the zero field energy separation.
The parameter is given by
Physically, is the ratio of the paramagnetic interaction (the "Zeeman energy") to the hyperfine separation. The Breit-Rabi energy level diagram for hydrogen and deuterium are shown in the figure. The units reflect current interest in atom trapping. Low-field quantum numbers are shown. It is left as an exercise to identify the high field quantum numbers.
Energy level structure for a single-electron atom with nuclear spin I = 1/2, such as hydrogen (left), and I = 1, such as deuterium (right). Note the typo on the left: The top state is F=1 not F=2. From Molecular Beams by N.F. Ramsey [2].
References
- Lande1923: A. Landé. Zeitschrift für Physik, 15:189, 1923.
- Ramsey1956: Norman F. Ramsey. Molecular Beams. Clarendon Press, 1956.
- Uhlenbeck1926: G. E. Uhlenbeck and S. Goudsmit. Spinning electrons and the structure of spectra. Nature, 117(2938):264, Feb 1926.





![{\displaystyle {\bf {L}}\equiv {\bf {r}}\times {\bf {p}}=m[{\bf {r}}\times {\bf {v}}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9ec2eddb604bfaa8fd59d39605c478c24e75b46e)
![{\displaystyle {\bf {\mu }}\equiv {\frac {1}{2}}{\bf {r}}\times {\bf {i}}={\frac {q}{2}}[{\bf {r}}\times {\bf {v}}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6aaadcf4f4cf0a3b5f7ab0f58413a6f6e0b1c6a2)






























![{\displaystyle \Delta E_{z,m,-1}={\left[g_{j^{\prime }}m-g_{j^{\prime \prime }}(m-1)\right]}\mu _{B}B={\left[(g_{j^{\prime }}-g_{j^{\prime \prime }})m-g_{j^{\prime \prime }}\right]}\mu _{B}B}](https://wikimedia.org/api/rest_v1/media/math/render/svg/80d5db3f438ad77862cfde1c87a01e4c2f17232b)




















![{\displaystyle H_{z}=-\mu _{B}{\left[-g_{j}({\bf {J}}\cdot {\bf {F}})+g_{I}({\bf {I}}\cdot {\bf {F}})\right]}{\frac {{\bf {F}}\cdot {\bf {B}}_{0}}{{\left|{\bf {F}}\right|}^{2}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6563fd93a29a291e597bb2df56da7d1be62621dc)